Medical misdiagnoses continue to be a significant concern worldwide, often leading to unnecessary complications and preventable deaths. According to the World Health Organization (WHO), at least 5% of adults in the U.S. experience a diagnostic error annually. The impact on a global scale is even more alarming. Despite rapid advancements in Artificial Intelligence (AI) and Machine Learning (ML), adoption in clinical settings remains limited. Many healthcare professionals remain skeptical, with only 3% of European healthcare organizations expressing trust in AI-enabled diagnostics. This blog explores the application of Neural Networks in breast cancer detection using the Wisconsin Breast Cancer Dataset. It examines how TensorFlow based models can improve diagnostic accuracy and assesses the potential of AI-driven systems in medical practice

Have you felt rushed in a doctor’s office? Have you ever left an appointment wondering if the doctor thoroughly reviewed your blood test results and other relevant information? Have you doubted the Doctor’s opinion? You are not alone!
In a 2019 World Health Organization (WHO) article, WHO states that their research shows that at least 5% of adults in the United States experience a diagnostic error each year in outpatient settings. In a 2023 article in BMJ, the authors state that there are 2.59 million missed diagnoses in the US, accounting for 371,000 deaths and 424,000 disabilities. These numbers are for only the false negative errors. When considered on a global scale, the numbers are staggering.
Whatever may be the reason for the errors in medical diagnosis, it’s obvious that these numbers must come down. Most doctors that I have met for a professional consultation, for myself or my family members, have advised me not to ‘Google’ medical conditions. At the same time, they do not have enough of time or patience to explain the condition. I can’t blame them, considering their patient load and time constraints.
The enormous interest in AI and Machine Learning, in all walks of life, is a tool that doctors should be using daily to minimize errors in medical diagnosis. I had assumed that this is happening at a rapid pace. But I was so wrong on this. In a 2022 article in the Frontiers in Medicine, the authors conclude that from their survey of medical professionals in 39 countries, 38% had awareness of clinical AI, but that 53% lacked basic knowledge of clinical AI. Their work also revealed that 68% of doctors disagreed that AI would become a surrogate physician, but they believed that AI should assist in clinical decision making. In a 2024 online summary, it is mentioned that 42% of healthcare organizations in the European Union were currently using AI technologies for disease diagnosis, but that only 3% trusted AI-enabled decisions in disease diagnostics. These pieces of information only indicate that the adoption of AI for disease diagnosis is under suspicion by the professionals. If anything, the adoption is slow, though the advancement in AI and Machine Learning has been very rapid. There is a trust and acceptance deficit when it comes to AI/ML in medical practice. Integration of AI/ML into clinical workflows would be the next big challenge. Finally, regulatory approvals would be a barrier to AI/ML implementation in medical establishments. But these hurdles will be overcome in due time, hopefully sooner rather than later.
I like to work on small cases when confronted with big questions such as this one. I’ll share with you a case that is based on Breast Cancer. American Cancer Society estimates that Approximately 1 in 8 women in the US (13.1%) will be diagnosed with invasive breast cancer, and 1 in 43 (2.3%) will die from the disease. Breastcancer.org estimates that approximately 310,720 women are expected to be diagnosed with invasive breast cancer annually in the US. Stopbreastcancer.org estimates that the mortality rate in the US is about 42,170 annually. WHO reports that in 2022 approximately 2.3 million women worldwide were diagnosed with breast cancer, accounting for 11.6% of all cancer cases globally. Further, it reported 670,000 breast cancer related deaths in 2022.
Doctors use a variety of techniques to detect breast cancer – mammography, breast ultrasound, PET scans, DNA sequencing and biopsies. A biopsy, which is a small extraction of a physical sample for microscope analysis, is a standard investigation tool. The investigations are performed by pathologists. The output from this analysis are measurements and metrics that capture features, giving the pathologists a means to reliably diagnose whether the lesions are malignant or benign.
A reputed biopsy database, based on the fine needle aspiration technique, is the Diagnostic Wisconsin Breast Cancer Database. It contains data for 569 patient biopsies, with each data set having 30 measurement features, shown here.
id, diagnosis, radius_mean, texture_mean, perimeter_mean, area_mean, smoothness_mean, compactness_mean, concavity_mean, concave_points_mean, symmetry_mean, fractal_dimension_mean, radius_se, texture_se, perimeter_se, area_se, smoothness_se, compactness_se, concavity_se, concave_points_se, symmetry_se, fractal_dimension_se, radius_worst, texture_worst, perimeter_worst, area_worst, smoothness_worst, compactness_worst, concavity_worst, concave_points_worst, symmetry_worst, fractal_dimension_worst
The header contains 32 categories, but the first column is the patient ID and the second column is the actual diagnosis, M is for malignant and B is for benign. Excluding this header and the first 2 columns, the data is a matrix of size (569,30). With 30 pieces of input data for a single biopsy for a patient, it seems daunting for a pathologist to look at all of them, in its entirety, to diagnose whether a biopsy is cancerous or not. For example, the large input feature set for the first patient, based on actual data in the data set, is shown here to give you an idea of the volume of data to consider before a diagnosis.
842302,M,17.99,10.38,122.8,1001,0.1184,0.2776,0.3001,0.1471,0.2419,0.07871,1.095,0.9053,8.589,153.4,0.006399,0.04904,0.05373,0.01587,0.03003,0.006193,25.38,17.33,184.6,2019,0.1622,0.6656,0.7119,0.2654,0.4601,0.1189
Using this dataset, a Neural Network algorithm for Structured Machine Learning was created, using TensorFlow. The Jupyter Notebook Python code is on Github. The Neural Network consists of 3 hidden layers, the first one with 25 neurons (units), the second one with 15 neurons and the third one with 1 neuron. The first two layers use the ReLU function, while the last one uses the Sigmoid function. The architecture is shown here.
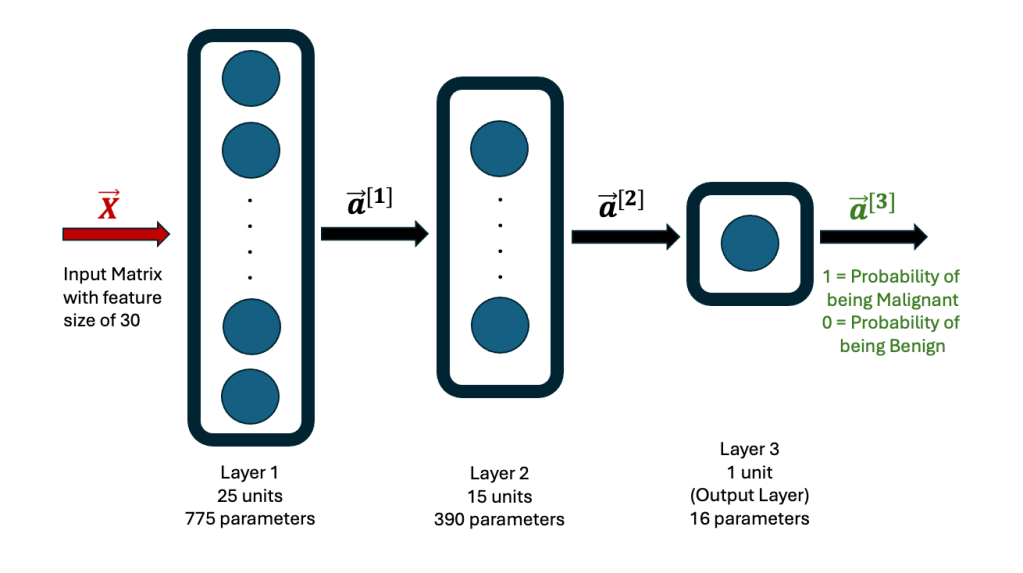
Rows 26 to 569 in the breast cancer data set were used as the Training set, while the first 25 rows were used as the Test set. The former is used to establish the weights and biases in each neuron in the network. The final output is either a 1 or 0, with 1 indicating that the data corresponds to a malignant diagnosis, while a 0 corresponds to a benign diagnosis.
After running the Neural Network code, the model was used to predict outputs for the entire Training set. Since the Training set contains the actual diagnosis (1 = M = Malignant) and (0 = B = Benign), it can be compared to the predicted output, to compute the accuracy of the Neural Network model. The model predicts a 99.26% accuracy. The predicted versus the actual output for the first 25 rows of the Training set is shown here. For the 15th row, the model predicts the outcome as 0, while the actual outcome is 1. Hence, the overall accuracy over the entire Training set is less than 100%, but still remarkable at 99.26%.

Next, the same model is used to predict the outcome for the Test set. The model has never seen this Test set before. It is equivalent to new patient data coming from the field. The prediction from the model for the Test set shows an accuracy of 100%! For comparison, the entire 26 rows of the predicted versus actual outcomes for the Test set is shown here.

These results are stunning. It emphatically shows the power of Machine Learning algorithms. For this specific case study, with a Training set of 543 patient records, it is possible to predict the cancer diagnosis for any new patient record, with an extremely high degree of accuracy.
With the number of tests that doctors ask patients to go through, hundreds of data values are generated. To make sense of all these data values, data analytics is required, rather than reliance on a cursory glance by a doctor. Neural Networks and Supervised Machine Learning are powerful AI tools that will benefit the patient today. AI can be applied to any disease diagnosis, for which raw data exists. Its adoption for reliable medical diagnosis is the need of the hour.
For those interested, the breast cancer dataset can also be analyzed using a Logistics Regression algorithm, using the Scikit-learn package. This code has also been provided on Github. The results are comparable to the Neural Network algorithm. Another small note – the TensorFlow package is one among several options available for writing Neural Networks code. Other choices are PyTorch (Meta), JAX (Google), MXNet (Apache) and CNTK (Microsoft).
You can take the Model for a test spin on the Hugging Face Platform – Breast Cancer Neural Network Prediction